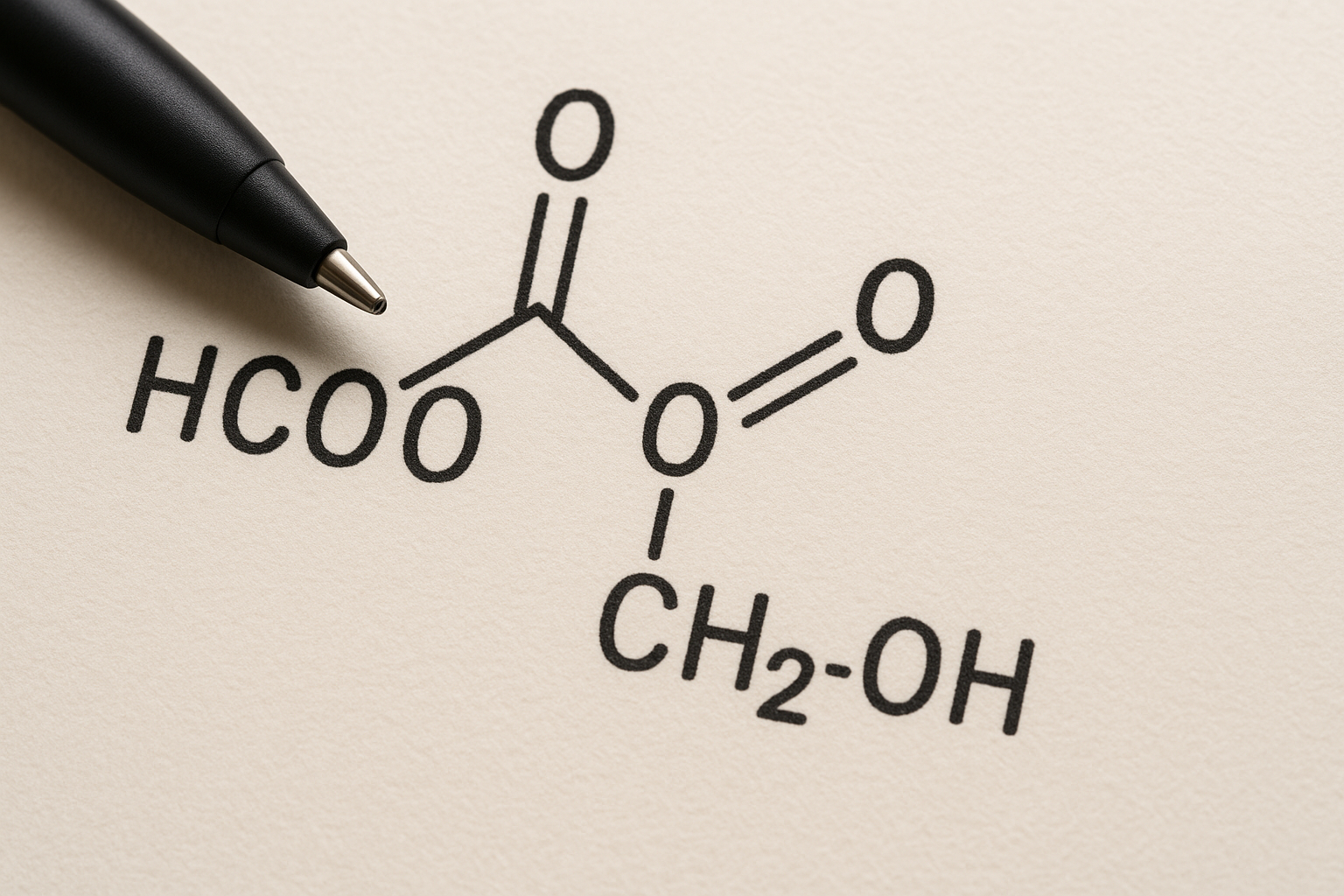
Introduction to HCOOCH CH2 H2O
Chemistry often introduces us to complex formulas that represent fascinating molecules with unique behaviors. One such example is HCOOCH CH2 H2O, a compound that brings together structural complexity and interesting chemical properties. To understand this compound, it is important to break down its components, analyze its structure, and explore its real-world significance. This guide will walk through its molecular makeup, bonding, and role in chemistry, making the concept easy to grasp for students, researchers, and curious learners alike.
Understanding the Molecular Formula
At first glance, the formula HCOOCH CH2 H2O may appear confusing. It combines functional groups that suggest the presence of a formate ester along with water molecules. The formula points toward an interaction between organic and inorganic components, which makes it worth studying. By carefully analyzing the notation, we can uncover how its structure influences its properties and applications.
Structural Breakdown of HCOOCH CH2 H2O
The structural backbone of HCOOCH CH2 H2O involves a formate group (HCOO–) linked with a methylene (CH2) unit, along with water as a possible hydrate or stabilizing element. Such arrangements often exist in organic chemistry where esters or hydrated derivatives form. Understanding this structure is essential because the placement of atoms and bonds directly determines the reactivity and stability of the compound.
Bonding and Functional Groups
Functional groups define the chemical personality of a molecule. In HCOOCH CH2 H2O, we see hints of ester-like behavior combined with hydrogen bonding from the water component. The carbon-oxygen bonds suggest polar interactions, while the methylene group contributes hydrophobic tendencies. This balance between polar and non-polar features makes the compound potentially versatile in chemical reactions.
Physical Properties of HCOOCH CH2 H2O
Like many organic derivatives, the physical properties of HCOOCH CH2 H2O—such as solubility, boiling point, and stability—are strongly influenced by hydrogen bonding and molecular weight. The attached water molecule may enhance solubility in polar solvents, while the organic portion gives it compatibility with hydrophobic environments. These mixed properties make it an interesting compound in experimental setups.
Chemical Properties and Reactivity
The reactivity of HCOOCH CH2 H2O is shaped by the presence of oxygen-rich groups. It may undergo hydrolysis, esterification, or oxidation depending on the conditions. Water in its formula suggests possible hydration or coordination chemistry, which could make it responsive to changes in pH or temperature. Such features often determine how chemists apply the compound in laboratory or industrial contexts.
HCOOCH CH2 H2O in Organic Chemistry
In the realm of organic chemistry, esters and hydrated compounds like HCOOCH CH2 H2O play a central role. They are often studied for their structural diversity and functional importance in reactions. This compound may serve as a model for understanding how formates interact with other organic groups, making it relevant for students learning about functional groups and reaction mechanisms.
Experimental Significance
Compounds such as HCOOCH CH2 H2O are valuable in experiments where controlled reactivity is needed. The combination of polar and non-polar characteristics can make it useful in solubility testing, reaction medium design, or synthesis pathways. Chemists often explore such compounds to discover new ways of stabilizing molecules or improving reaction yields.
Industrial and Practical Relevance
Though not as widely known as mainstream chemicals, derivatives related to HCOOCH CH2 H2O may find relevance in specific industries. Esters and hydrated organic compounds often serve as intermediates in pharmaceuticals, solvents, and material sciences. By understanding their behavior, researchers can tailor them for specialized applications where controlled reactivity is required.
Cultural and Educational Impact
Beyond laboratories, compounds like HCOOCH CH2 H2O carry educational value. They help students appreciate how chemical notation represents complex realities and how simple-looking formulas can reflect deep chemical interactions. Learning about such molecules enhances analytical skills, encouraging learners to think critically about chemical relationships.
Comparison with Related Compounds
To fully appreciate HCOOCH CH2 H2O, it helps to compare it with similar compounds. Other formates, esters, and hydrates exhibit comparable features, such as hydrogen bonding and solubility variation. This comparative study highlights the importance of functional group placement and demonstrates how slight structural changes can significantly affect chemical behavior.
Stability and Safety Considerations
Like many organic derivatives, HCOOCH CH2 H2O may require careful handling. Its stability depends on environmental factors such as temperature, exposure to air, and pH conditions. Water in its formula may enhance stability in some cases, but hydrolysis or decomposition can occur under unfavorable conditions. Safety protocols in laboratories emphasize controlled storage and proper disposal to avoid unwanted reactions.
Research Opportunities with HCOOCH CH2 H2O
For researchers, HCOOCH CH2 H2O presents an opportunity to explore hybrid organic-inorganic interactions. Its structure makes it a candidate for studies in catalysis, green chemistry, and molecular design. With growing interest in sustainable solutions, understanding how such compounds behave may lead to innovations in energy, medicine, or material development.
Conclusion
The formula HCOOCH CH2 H2O is more than just a chemical representation—it reflects the fascinating interplay of structure, bonding, and reactivity. From its functional groups and hydrogen bonding potential to its educational and industrial value, the compound offers key insights into the world of chemistry. Studying such molecules deepens our appreciation for how small variations in structure can unlock new properties and applications. For students, researchers, and professionals alike, exploring HCOOCH CH2 H2O is a reminder of how chemistry connects science, discovery, and innovation.